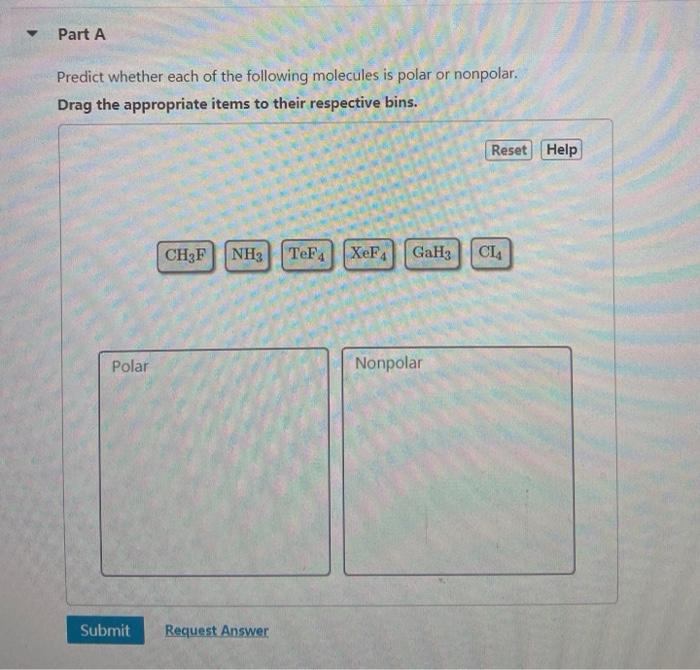
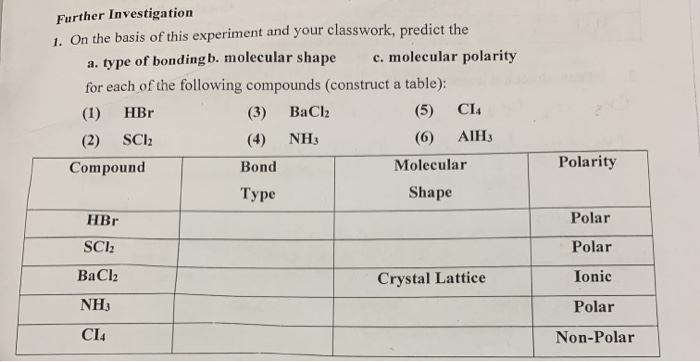
SOLVED: Choose the compound below that contains at least one polar covalent bond, but is nonpolar HCN IF3 SeF4 CBr4 Both B and C are nonpolar and contain a polar covalent bond.

Which of the following molecules can be nonpolar? (Select all that apply.) - cis-CHBr=CHBr - CH2=CH2 - cis-CHCl=CHCl - trans-CHCl=CHCl - none of the above | Homework.Study.com

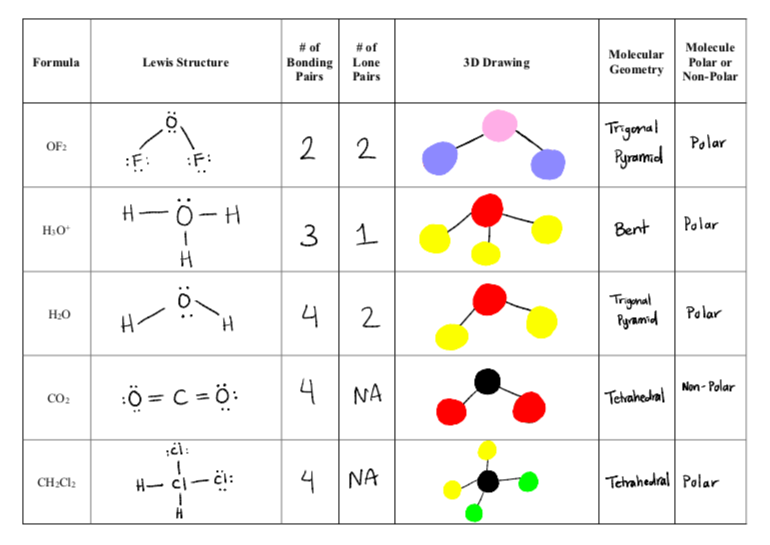
SOLVED: In the introduction for this experiment, we will characterize the following molecules as polar or nonpolar and determine if they are capable of hydrogen bonding with another molecule of the same

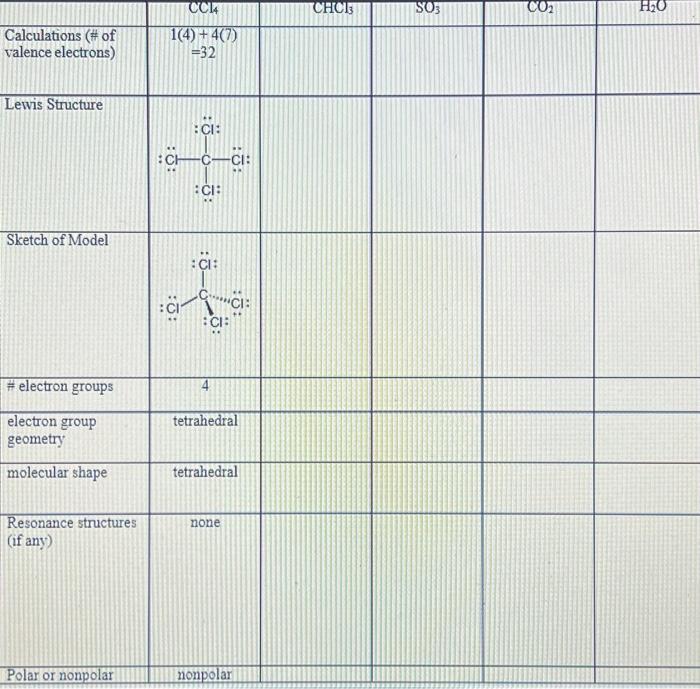
Draw the Lewis structure and predict the molecu- lar geometry of the following molecules: a. SO2 b. CI4 c. - brainly.com

Which solvent, water or carbon tetrachloride, would you choose to dissolve each of the following? a. KrF_2 b. SF_2 c. SO_2 d. CO_2 e. MgF_2 f. CH_2O g. CH_2=CH_2 | Homework.Study.com

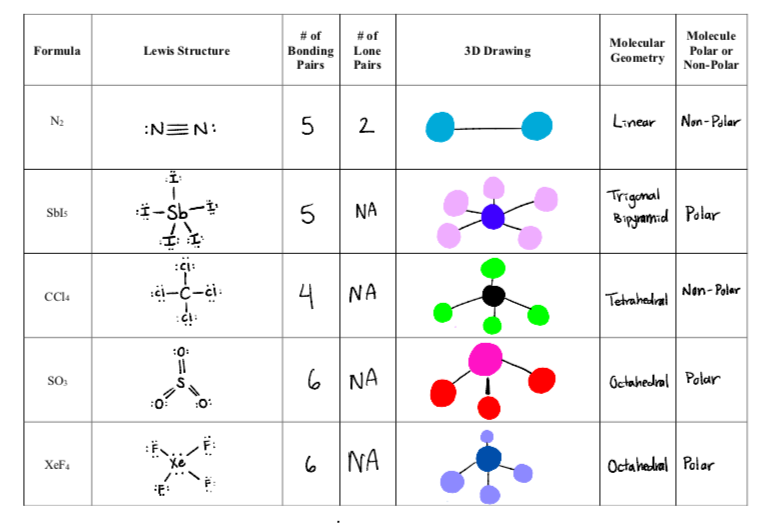
PPT - Bonds can be classified as being either polar or non-polar . PowerPoint Presentation - ID:9663902
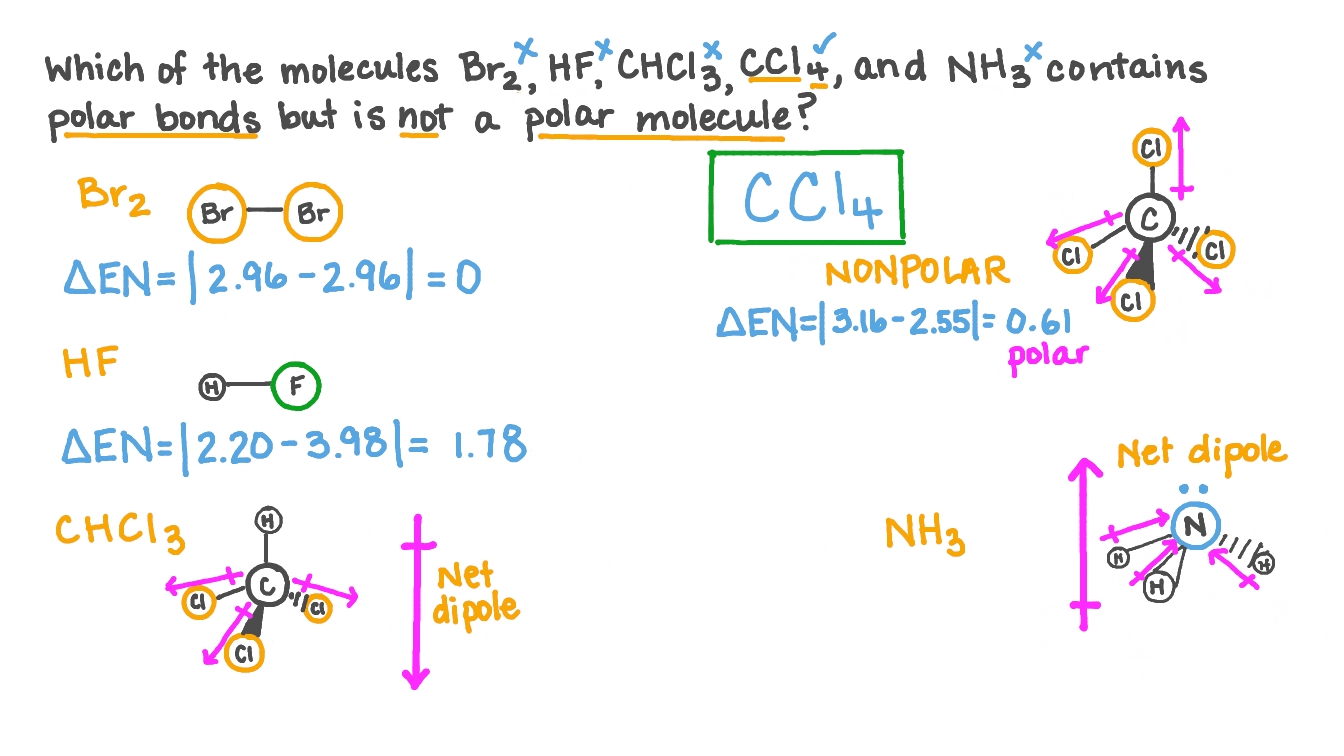
Question Video: Determining the Molecule That Contains Polar Bonds but Is Not a Polar Molecule | Nagwa

Which type of intermolecular force ("interparticle force") is the most important in CI4(s)? 1. Ionic bonds 2. Dipole-dipole forces 3. Hydrogen bonds 4. Ion-dipole forces 5. London Dispersion | Homework.Study.com
Which compound among CCL4 (carbon with chlorine) and CI4 (carbon with iodine) is more covalent and why? - Quora