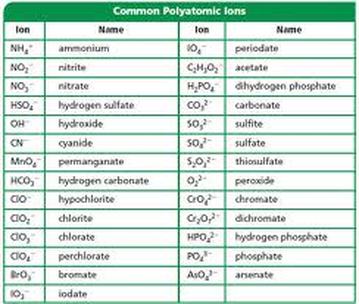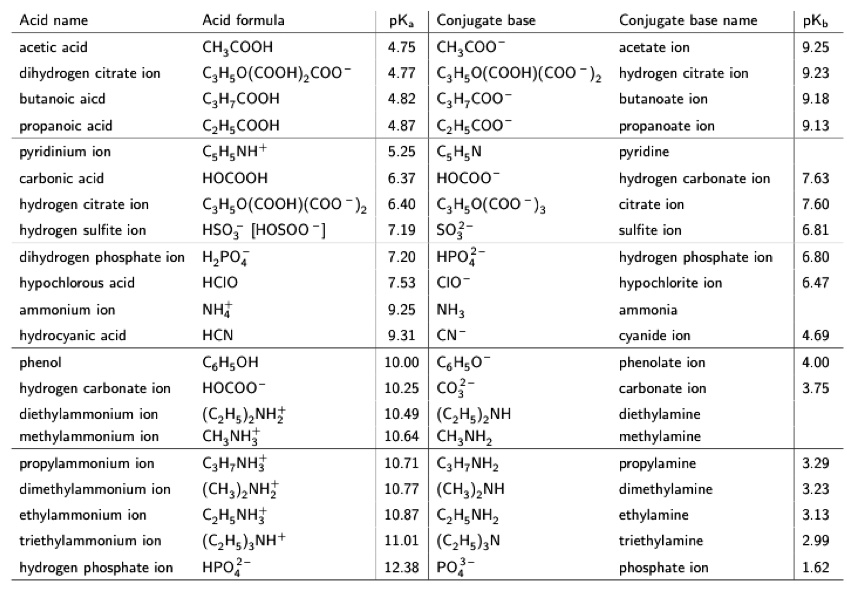
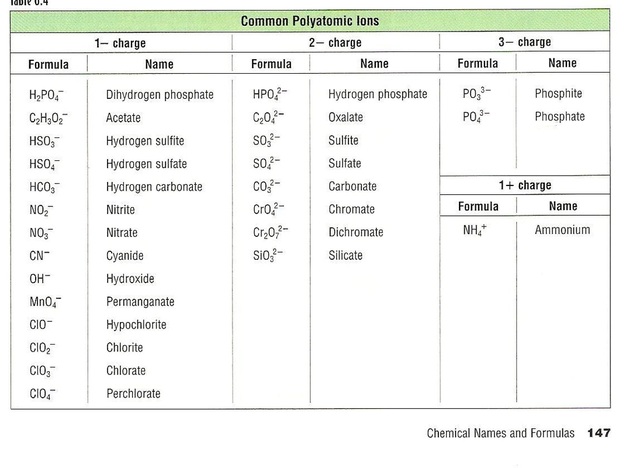
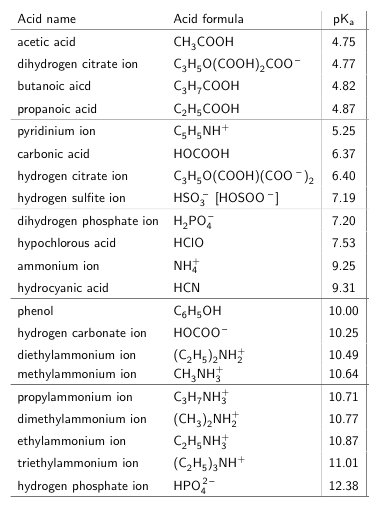
SOLVED: Text: Acid name Acid formula acetic acid CH3COOH dihydrogen citrate ion C6H8O7(COOH) COO- butanoic acid C4H8COOH propanoic acid C3H6COOH pyridinium ion C5H5NH+ carbonic acid H2CO3 hydrogen citrate ion C6H6O7(COOH)(COO-) hydrogen sulfite
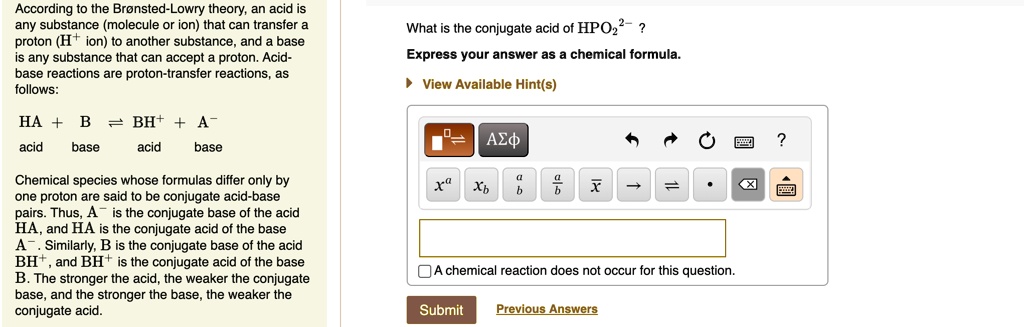
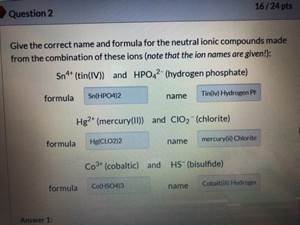
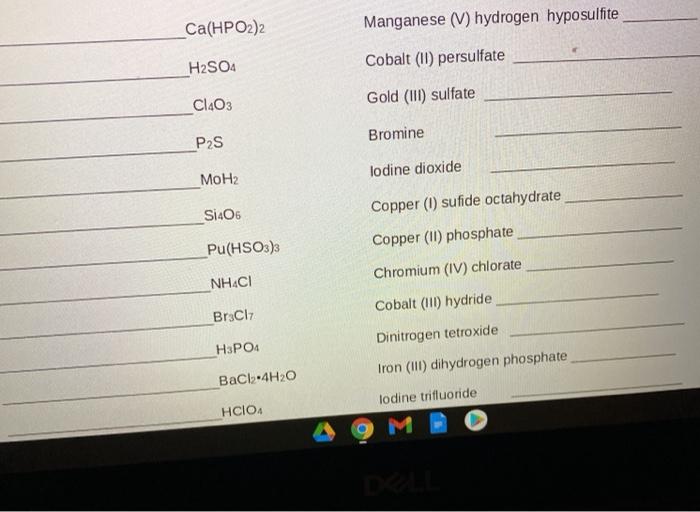
SOLVED: Texts: What is the conjugate acid of HPO2^2-? According to the Bronsted-Lowry theory, an acid is any substance (molecule or ion) that can transfer a proton (H+ ion) to another substance,








![HPO2]- HPO2]-](https://www.chemtube3d.com/images/gallery/inorganicsjpgs/HPO2-.jpg)
![Kannada] Conjugate base of H(2)PO(4)^(-) is Kannada] Conjugate base of H(2)PO(4)^(-) is](https://static.doubtnut.com/ss/web-overlay-thumb/9145078.webp)