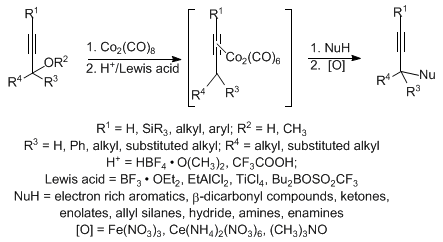Tetrahedron Letters | Vol 43, Issue 4, Pages 541-733 (21 January 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 27, Pages 4717-4893 (1 July 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 42, Pages 7451-7641 (14 October 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 41, Pages 7285-7449 (7 October 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 12, Pages 2127-2321 (18 March 2002) | ScienceDirect.com by Elsevier

Tetrahedron Letters | Vol 43, Issue 12, Pages 2127-2321 (18 March 2002) | ScienceDirect.com by Elsevier
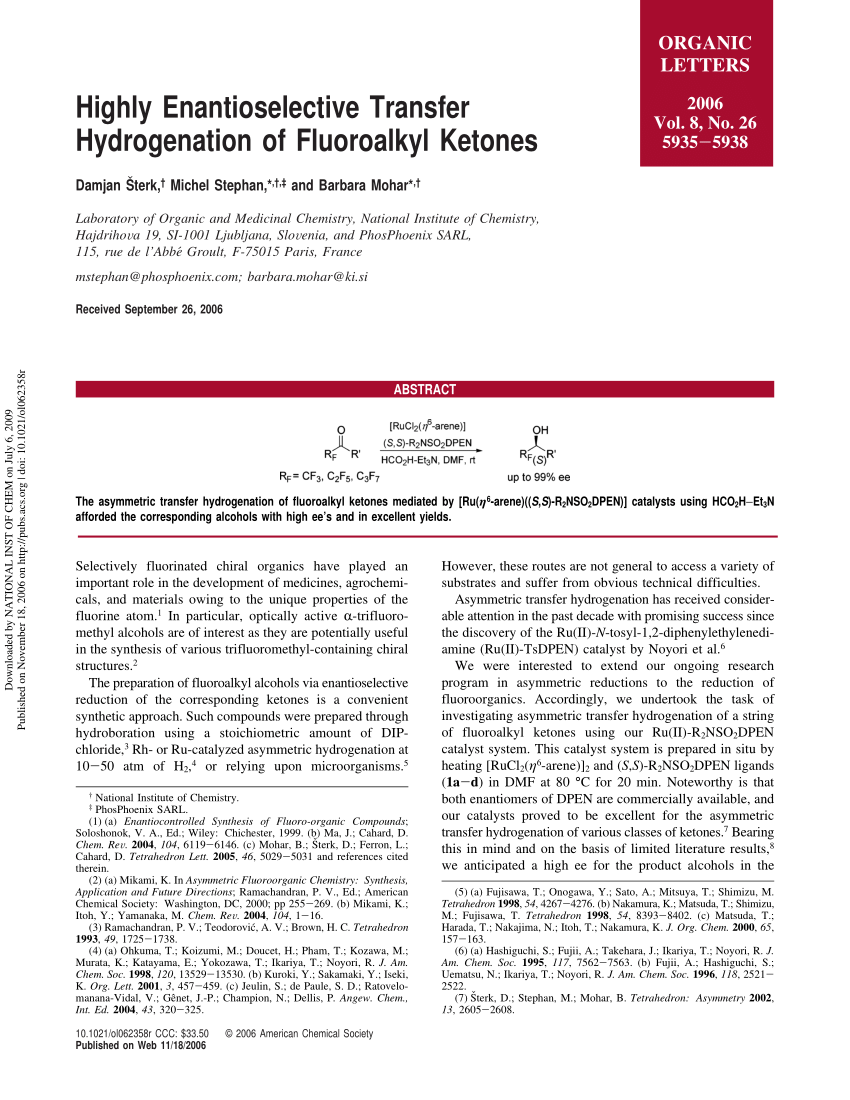
PDF) Stereoarrayed CF 3 -Substituted 1,3-Diols by Dynamic Kinetic Resolution: Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation
Synthesis Alerts is a monthly feature to help readers of Synthesis keep abreast of new reagents, catalysts, ligands, chiral auxi

Tetrahedron Letters | Vol 43, Issue 27, Pages 4717-4893 (1 July 2002) | ScienceDirect.com by Elsevier
Arylpalladium Phosphonate Complexes as Reactive Intermediates in Phosphorus-Carbon Bond Forming Reactions - UNT Digital Library